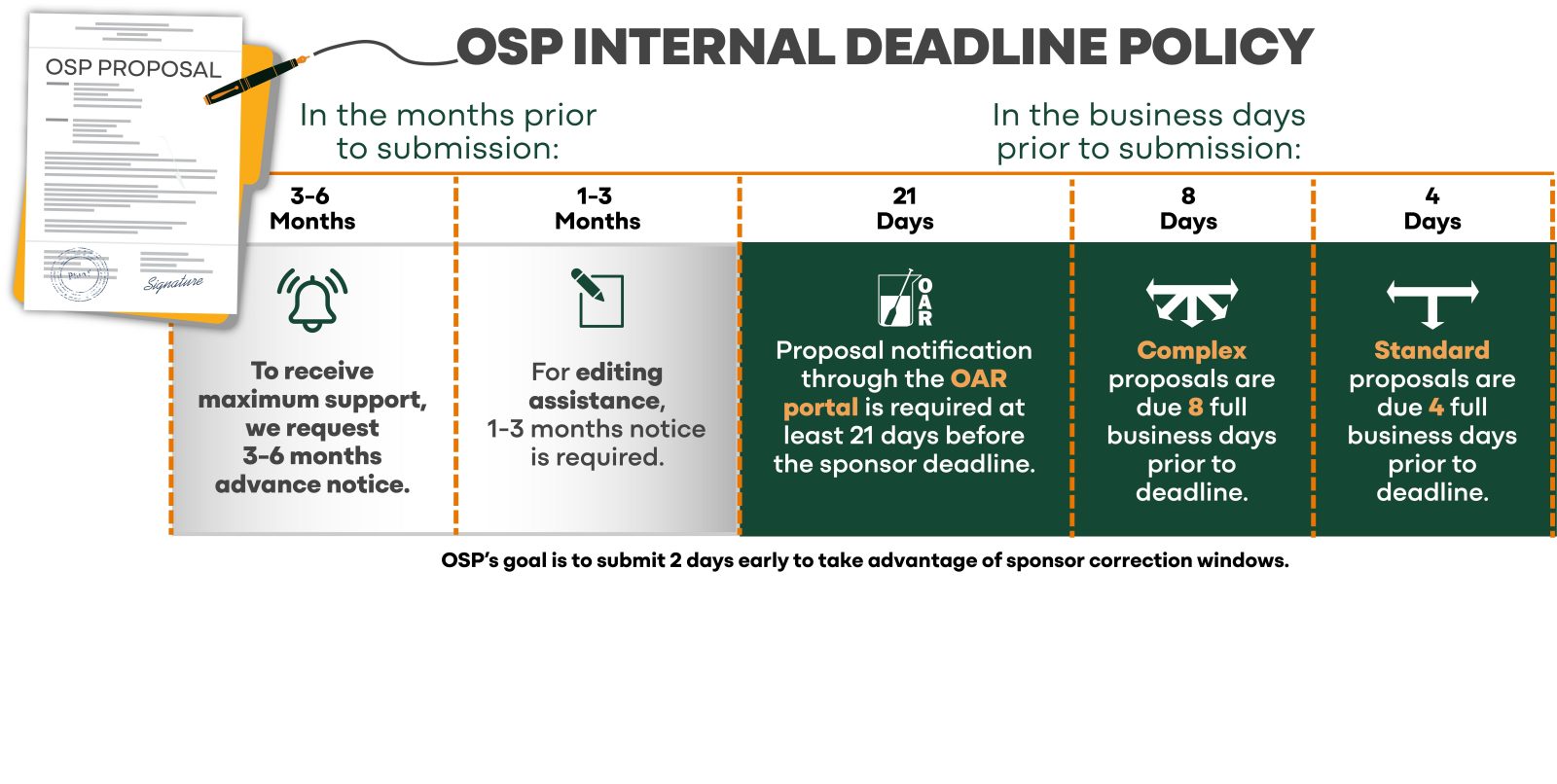
The Office of Sponsored Projects (OSP) and faculty must work together to ensure quality, compliant submissions with the end goal of increased federal awards. This can only happen when enough time is afforded to build the collaborative relationship necessary to accomplish the shared objective of an on-time submission of a complete and high-quality proposal. Faculty must notify OSP through the OAR portal of potential proposal submissions as early as possible, but no later than 21 days prior to submission. Please note that the deadlines are the dates by which ALL proposal items should be provided to OSP and not initial notification of a proposal.
- Standard proposal: due to OSP four (4) full business days prior to deadline.
- Complex proposal: due to OSP eight (8) full business days prior to deadline. A proposal qualifies as complex if any of the following are present: a subcontractor, foreign component, cost share, international sponsor, EER-E/ARPA-E, NEH/NEA, NIJ funding, or binding terms and conditions. In addition, complex proposals include NIH Common Fund, U or P type submissions or center level programs from any federal sponsor.
A complete proposal package includes all proposal components – both technical and non-technical, all forms required by the sponsor, any commitment letters from external parties plus required internal institutional letters, subcontract forms and budgets if applicable, and a cert record approved by all project personnel. For complex proposals, the technical documents must be in a complete form for format and compliance review. The principal investigator may make final edits until 4 full business days before the deadline; however, such changes should not impact the budget, institutional or collaborator commitments, or non-technical documents.
Proposals that do not adhere to the policy will not be submitted.
FAQ
- Immediately but no later than 21 days before the deadline. Create a record in the OAR portal even if you are only contemplating a proposal. Advance notice allows a more involved collaboration between you and the specialist, resulting in a fully complete and quality proposal. It is imperative for OSP to have prior knowledge of upcoming submissions to plan schedules and verify sponsor guidelines.
- The ideal notification would be a summary list of potential submissions sent at least every semester.
- The submission deadline is no later than 5pm on the date the proposal is to be submitted to UTD’s sponsor.
- When submitting directly to a sponsor, it will be the date listed in the solicitation. When UTD is a subawardee, the deadline will be the day the prime institution requests the documents.
- For submissions without a firm deadline or with a desired deadline prior to the sponsor deadline, the desired submission date should be used to determine the internal deadline.
- The complete application must arrive 4 or 8 full business days prior to the sponsor deadline. Holidays and University closures are not counted as business days.
- Example: If the deadline is a Friday, the proposal must be in OSP the previous Friday for a standard proposal (4 full business days) and the Monday prior for a complex proposal (8 full business day). See the chart below.
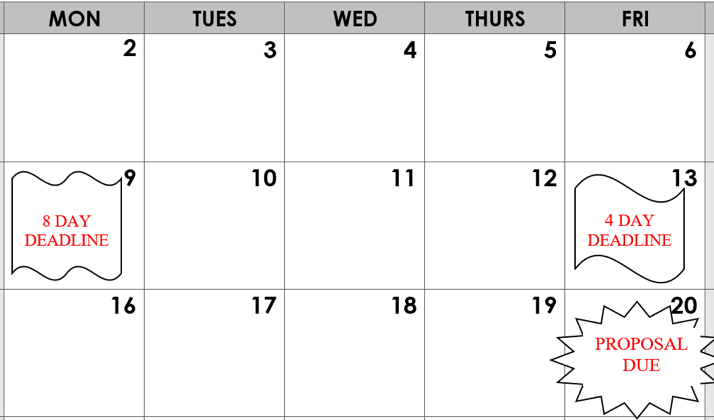
- OSP will submit the application as soon as it is ready to be submitted. Depending on the number of changes necessary, it could be submitted on the day it arrives to OSP or any day up until the deadline.
- OSP strives to submit 2 days prior to the deadline to take advantage of correction windows offered by sponsors.
- OSP will prioritize the proposals as they arrive based on a “first come, first served” model.
- Unlike standard proposals, a complex proposal will contain any of the following: a subcontractor, foreign component, cost share, international sponsor, EER-E/ARPA-E funding, or binding terms and conditions. In addition, complex proposals include NIH Common Fund, U or P type submissions or center level programs from any federal sponsor.
- We have a variety of grant proposal editors available with a track record of success in supporting faculty. We have partnered with Hanover Research, a grant development firm, to provide grant proposal editing/reviewing resources and to help faculty prepare competitive proposals.
- To learn more about the services provided and make a request, visit the webpage.
- Technical documents describe the specific science/engineering of the project that is proposed.
- Sponsors use a variety of names for these documents. They include Narrative, Summary, Abstract, Project Description, References, Human Subjects, Animals, and any other required scientific documents.
- Thorough review of these documents is needed to confirm compliance with all areas such as export control, human subjects, research security, data management, conflicts of interest, and preservation of fundamental research rights.
- These documents include biographical sketches, current and pending, collaborators and other affiliations, equipment, facilities, budget (including any required task/phase breakouts), budget justification, cost share, letters of commitment or support from collaborators and consultants – internal and external, all subaward documentation (consisting of a minimum – letter of commitment, budget/justification, and scope of work), multi-pi plan, data/resource sharing plans, postdoctoral mentoring plans, and any other required non-scientific documents.
- Each sponsor and solicitation may require additional non-technical, financial, and supplemental documents so it is important to read the proposal preparation guidelines for each proposal.
- All documents (needed by the sponsor and /or the University) must be received from the external entities Sponsored Programs Office (or equivalent) and provided to OSP 8 full business days before the deadline.
- Documents cannot be accepted from the individual collaborators and must be officially submitted by the institution.
- It is important to notify collaborators early so they can meet their own, as well as OSP’s internal deadlines.
- Since the lead institution uploads the Project Description, the document is not required to be finalized at the 4 business day mark.
- If UTD is a proposed subrecipient, the deadline will align with the prime recipient’s institutional deadlines, which may be well before the sponsor deadline.
- The lead institution’s deadline will be used as the due date in the cert portal and used to create the internal timeline for review and submission.
- All documents must be provided to the lead institution by OSP.
- Include a technical and administrative contact for the lead institution in the cert portal so OSP can begin communications quickly. Include any previous communications with the lead institution.
- Any pre-proposal that requires institutional approval must follow the policy.
- Common indicators of needing institutional approval are references to the university’s research office (AOR/SRO/SPO) needing to review, approve, submit, sign, etc.
- Common language seen in solicitations are, but not limited to:
- “Institutional countersignature” required
- “I certify that I am authorized to submit”
- “I have reviewed and agree to terms and conditions…
- “Pre-proposals” that require a higher degree of review (e.g., coordination with other university offices, joint faculty or national laboratory involvement, line-item budgets, cost share, personnel documents such as biosketches, current & pending support, conflict of interest review, etc.) must follow the standard policy.
- Most funding opportunities, especially federal, have published deadlines such that the internal deadline policy can be followed.
- If the solicitation is issued with a short window, ORI will determine if there are extenuating circumstances beyond the PI’s control.

You must be logged in to post a comment.