
Laser Scanning Confocal Microscope equipped with 7 laser lines, a fully automated stage for whole tissue stitching, and live cell imaging capability
Location
Visuals
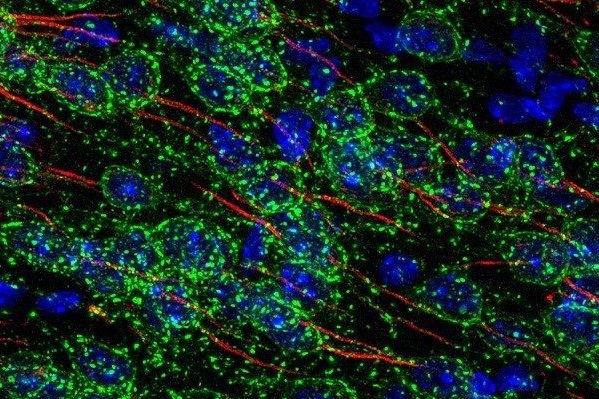
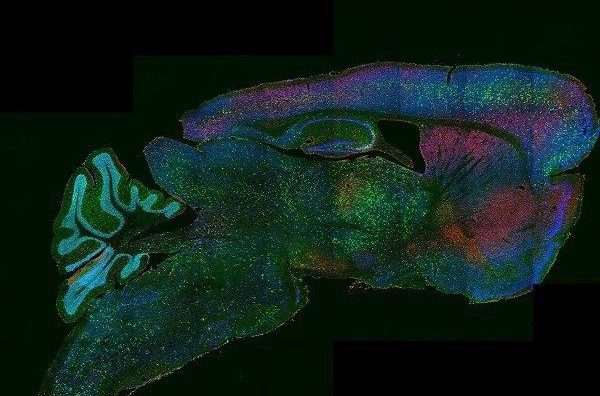
Capabilities
- Imaging diverse fluorophores in fixed or live samples
- Temperature, humidity, CO2 control
- Spectrally tunable detectors
- Ultra-fast resonant scanning (reduced photo-damage with live samples)
- 3D z-stacks, timelapse, multi-position timelapse
- FRAP/FLIP/Photoactivation, FRET (ratio or acceptor photobleaching)
Cost
Internal user $32/hour
Software Manual
Module 1: Basic Introduction, Layout, Dye, and Detector Selection
Module 4: Image Acquisition, Z and Time Series, Data Saving
Module 5: Basic Image Processing
Detailed Specifications
Microscope
- Olympus IX83 fully motorized inverted microscope
- Zero Drift Compensator (IX3-ZDC2) laser-based autofocus
- Ultrasonic automated stage and multiple area time lapse stage control software, as well as well-plate navigator tool for multi-area, time-lapse, and multi-area mosaic image stitching
- Tokai hit stage top, live-cell incubation chamber
- Analog and digital in-out box for synchronization with external devices
- Facilitates integration of PicoQuant, FCS/FCCS, and FLIM hardware packages
Tandem Scanners
- Conventional scanner for high-definition imaging
- Resonant scanner for high-speed imaging (capable of 30 FPS@512×512 and 438 FPS@512×32)
Light Sources
- All Diode Lasers with 7 Laser Lines – 405 nm, 445 nm, 488 nm, 514 nm, 561 nm, 594 nm, and 640 nm
- LED-based transmitted and epifluorescence illumination
Conventional Fluorescence Filters
- Dapi
- Green
- Red
Objectives
Air objectives:
- PLAPON1.25X; PLAN APO 1.25X, NA 0.04, WD 5.1 mm
- UPLSAPO10X2; U PLAN S-APO 10X, NA 0.4, WD 3.1 mm
- UPLSAPO20X; U PLAN S-APO 20X, NA 0.75, WD 0.65 mm
Silicone oil objectives:
- UPLSAPO30XS; UPLSAPO N 30X SI OIL, NA 1.05, WD 0.8mm, w/CC (with correction collar)
- UPLSAPO40XS; UPLSAPO N 40X SI OIL, NA 1.25, WD 0.3mm, w/CC
- UPLSAPO100XS; UPLSAPO 100X SI OIL, NA 1.35, WD 0.20mm, w/CC
Silicone oil provides a better refractive index match to cells and tissue than water or traditional immersion oil for brighter and deeper imaging. Silicone Oil does not evaporate nor degrade, permitting long live cell/tissue time-lapse imaging with ease. Another benefit is greater working distance than many traditional immersion objectives while still retaining high numerical apertures (30x – 1.05 NA 800 µm WD; 40x – 1.25 NA 300 µm WD; 100x – 1.35 NA 200 µm WD)
The Plan Super Apochromat design fully compensates for both planar and chromatic aberrations from the UV to the near infrared region for precise multicolor colocalization imaging.
Dichroic(s)
- Conventional dichroic mirrors
Detectors
- 4 High-Sensitivity Peltier-Cooled GaAsP Spectral Confocal Detectors with Olympus TruSpectral High-Efficiency Volume Phase Hologram spectral detection system (1-100 nm adjustable emission bandwidth with 2 nm spectral resolution)
- Transmitted light detector for Brightfield/DIC Image Acquisition
Computer and Software
- HP Windows 7 64bit Confocal Workstation and 30” Monitor
- Complete Fluoview Acquisition & Analysis Software provides FRAP, FRET, Ratiometric Imaging Display & Analysis, 3D Image Acquisition & Display, Live 16 Channel Spectral Unmixing, Fluorescence Intensity Measurements, Time-Lapse Imaging, and more…
- Olympus cellSens Dimension provided for additional post-acquisition data analysis and processing
- Includes Olympus cellSens Count & Measure Solution for threshold-based object detection as well as automatic object measurement and classification
Includes Olympus cellSens 3D Deconvolution Solution utilizing Constrained Iterative Deconvolution algorithm for improved resolution, contrast, and dynamic range.

Spinning Disk Confocal Microscope designed for high speed live cell imaging
Location
Visuals
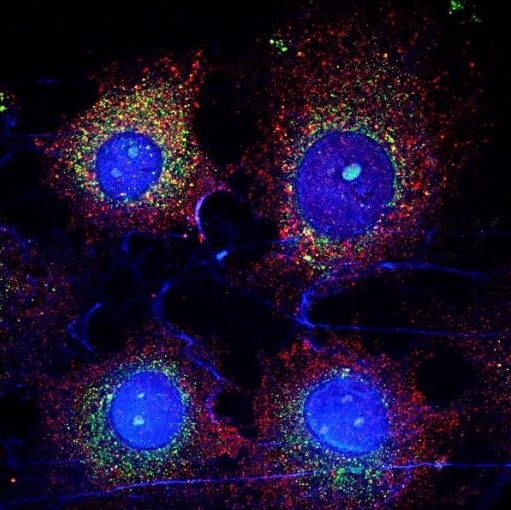
Capabilities
- High-speed multidimensional imaging with optical sectioning at speeds of up to 200 FPS
- Spinning disk modality produces high SNR images with minimal photobleaching and photodamage
- High sensitivity SCMOS camera
- Laser lines for all classes of fluorescent proteins
- Incubation for mammalian cells
- IR reflection based autofocus
- Silicon oil 100x objective for refractive match
- 120nm SIM Super-Resolution at 3 FPS
Cost
Internal user $32/hour
Software manual
PDF file can be found on this link.
Video tutorials can be can be here:
Module 1: Overview and Basic Image Acquisition
Module 2: Multidimensional Acquisition
Detailed Specifications
Microscope
- Olympus IX83 Fully Motorized Inverted Microscope
- Zero Drift Compensator (IX3-ZDC2) laser-based autofocus
- Ultrasonic automated stage, Multiple Area Time Lapse Stage Control Software, and Well-Plate Navigator Tool for multi-area, time-lapse, and multi-area mosaic image stitching
- Tokai hit stage top live-cell incubation chamber
Tandem Scanners (select at software initialization)
- Yokogawa W1 Spinning Disk for high-speed imaging up to 200 FPS
- Airy disk oversampling drives resolution down to 120 nm at 3 FPS
Light Sources:
- 405 nm: 50 mW, 488 nm: 100 mW, 561 nm: 100 mW, 640 nm: 100 mW
- X-CITE 120 LED-based epifluorescence illumination
Conventional Fluorescence Filters
- 87-059; long pass dichroic filter 325-435(R)/444-850(T)
- 67-072; long pass dichroic filter 440-500(R)/513-730(T)
- 67-075; long pass dichroic filter 520-585(R)/600-800(T)
Objectives
Air objectives
- UPLSAPO4X; U PLAN S-APO 4X, NA 0.16, WD 13 mm
- UPLSAPO10X2; U PLAN S-APO 10X; NA 0.4, WD 3.1 mm
- UPLSAPO20X; U PLAN S-APO 20X, NA 0.75, WD 0.65 mm
- UPLSAPO40X2; U PLAN S-APO 40X; NA 0.95, WD 0.18 mm
Silicone oil objectives
- UPLSAPO100XS; UPLSAPO 100X SI oil, NA 1.35, WD 0.20 mm
Silicone oil provides a better refractive index match to cells and tissue than water or traditional immersion oil for brighter and deeper imaging. Silicone Oil does not evaporate nor degrade, permitting long live cell/tissue time-lapse imaging with ease. Another benefit is greater working distance than many traditional immersion objectives while still retaining high numerical apertures (100x – 1.35 NA 200 µm WD).
The Plan Super Apochromat design fully compensates for both planar and chromatic aberrations from the UV to the near infrared region for precise multi-color colocalization imaging.
Dichroic(s)
- 49000-IX3; ET-DAPI D350/50X, BS400, 460/50M
- 49002-IX3; ET-EGFP/FITC/CY2 470/40X, BS495, 525/50M
- 49008-IX3; ET-MCHERRY/TXRED 560/40X, BS585, 630/75M
- 49006-IX3; ET-CY5 620/60X, BS660, 700/75M
Detectors
- Hamamatsu Orca Flash 4.0 Plus SCMOS Digital Camera
Computer and Software
- HP Windows 7 64-bit Confocal Workstation and 30” Monitor, 2TB HD, 128 & 256GB SSDs, 32GB DDR3 RAM
- Metamorph advanced Olympus imaging software

Total Internal Reflectance Fluorescence system equipped with 4 laser lines with individual control
Location
Capabilities
- Total Internal Reflection Fluorescence (TIRF) and Highly-Inclined Laminated Optical sheet (HILO) imaging
- Variable TIRF depth
- Temperature, humidity, CO2 control
- 4 channels with individual beam paths with FRAP optics for first line
- Beam splitter for simultaneous 2 color imaging
Cost
Internal user $32/hour
Detailed Specifications
- Olympus IX83 Fully Motorized Inverted Microscope
- Zero Drift Compensator (IX3-ZDC2) laser-based autofocus
- Ultrasonic automated stage and Multiple Area Time Lapse Stage Control Software, as well as Well Plate Navigator Tool for multi-area time-lapse and multi-area mosaic image stitching
- Tokai hit stage-top live-cell incubation chamber
Light Sources
- Laser diode 405 nm (100 mW), 488 nm (100 mW), 561 nm (100 mW) (DPSS), 640 nm (100 mW)
- X-CITE 120 LED-Based Transmitted and Epifluorescence Illumination
Conventional Fluorescence Filters
- Dapi
- Green
- Red
Objectives
Air objectives
- UPLFLN4xPH; U PLAN FLUORITE 4x phase, NA 0.13, WD 17 mm
- UPLFLN10x2PH; U PLAN FL 10x phase, NA 0.3, WD 10 mm
- UCPLFLN20xPH; C PLAN FL 20x phase, Correction collar, NA 0.7, WD 0.8-1.8 mm
- LUCPLFLN40xPH; LWD U PLAN FL 40x phase, Correction collar, NA 0.6, WD 2.7-4.0 mm
Oil objective for TIRF and HILO microscopy
- UAPON 100 x OTIRF; UAPO 100 x TIRF, NA 1.49, WD 0.1 mm
Dichroic(s)
- TRF49901-OL3; ET-405 nm LASER TIRF SET
- TRF49904-OL3; ET-488 nm LASER TIRF SET
- TRF49904-OL3; ET-488 nm LASER TIRF SET
- TRF49909-OL3; ET-561 nm LASER TIRF SET
- TRF49914-OL3; ET-640-647 nm LASER TIRF SET
- TRF89901-OL3; ET-405/488/561/640 nm Laser Quad TIRF
- W-View Gemini optical splitter for simultaneous smFRET detection
Detectors
- IXON ultra 897 EMCCD, 56 FPS, 512 x 512, 16 µm pixel size.
Computer and Software
- HP Windows 7 64 bit Confocal Workstation and 30” Monitor 2 x 1 TB HD, RAID 1, 32GB DDR3
- Olympus CellSens Integrated Software for Automated Multi-Dimensional Acquisition, Multi-Area Stitching, and Well Plate Scanning. Includes analysis for FRET and Real-Time Intensity Analysis.
- Software controlled laser incidence angle and penetration depth

100-Slide Scanning System for fully automated high throughput imaging in brightfield or fluorescence modes
Location
Visuals
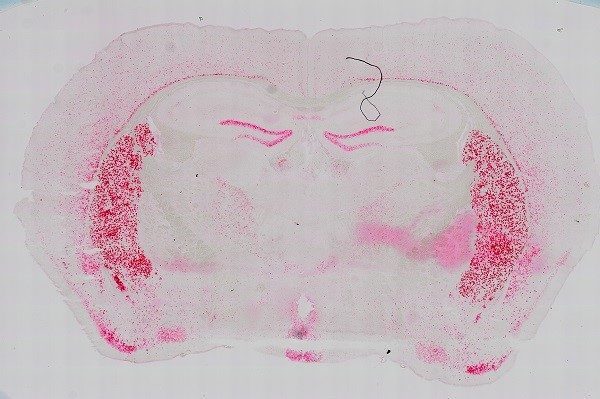
Capabilities
- High content imaging of fixed samples labeled with diverse stains and fluorophores mounted on slides
- Automated image stitching, 3D z-stacks for thick slide preparations
- Automatic specimen recognition capability increases data acquisition speed
- Semi-automated imaging of up to 100 slides at a time
Cost
Internal user $17/hour
Software Manual
Detailed Specifications
Microscope
- Olympus VS120 virtual slide scanner configured for transmitted and reflected light brightfield and epi-fluorescence scanning modes. Includes an automated slide loader for batch scans of 1-100 slides
Light Source
- SOLA-SE-II solid state white light excitation subsystem
- Built-in Koehler illumination for transmitted light
Dichroic(s)
- 49000-BX2; ET-DAPI D350/50X, BS400, 460/50M
- 49002-BX2; ET-EGFP/FITC/CY2 470/40X, BS495, 525/50M
- 49004-BX2; ET-CY3/TRITC 545/25X, BS565, 605/70M
- 49008-BX2; ET-MCHERRY/TXRED 560/40X, BS585, 630/75M
- 49006-BX3; ET-CY5 620/60X, BS660, 700/75M
Objectives
Air objectives
- 2x (NA 0.08) 7 μm/pixel
- 10x (NA 0.40) 66 μm/pixel
- 20x (NA 0.75) 0.33 μm/pixel
- 40x (NA 0.95) 0.17 μm/pixel
Detectors
- Hamamatsu Orca Flash 4.0 Plus Enhanced QE SCMOS Digital Camera
Computer and Software
- VS-ASW-FL V2.9 software for 5/6 slide and SL
- VS-HIGHSENS V2.9 license
- VS-RFAA for VS120

Intravital Multiphoton Fluorescence Microscope with dual multiphoton laser system for simultaneous scanning and excitation
Location
Visuals
Capabilities
- Multiphoton imaging of thick in vivo or in vitro samples
- Simultaneous photostimulation or dual-line imaging modes
- 405 nm UV photostimulation
- Long working-distance objectives optimized for multiphoton microscopy
- High-speed extended IR (1300 nm) multiphoton imaging (30 FPS at 512 x 512, 438 FPS at 512 x 32)
- 4 non-descanned detectors (2 GaAsP and 2 multi-alkali PMTs) capable of conducting Fluorescence Lifetime Imaging Microscopy (FLIM)
Cost
Internal user $33/hour
Software manual
Module 1: Basic introduction and lightpath
Module 2: Dye, detector, and laser selection
Module 4: Objectives and image acquisition
Detailed Specifications
Microscope
- Olympus IX83 fully motorized upright microscope
- Twin IR laser coupling optics with full laser enclosure for independent control of two IR lasers
- Motorized mirror assembly permits user-selectable stimulation + imaging or simultaneous dual-laser line excitation
- AOM laser attenuation (0%-100%, 0.1% increments) provides user selectable laser intensity adjustment and laser beam blanking to minimize photo-damage
- Fully-automated IR beam expander optimizes beam diameter to maximize image resolution with any selected objective
- Deep Focus Mode adjusts beam diameter in accordance with laser scattering sample conditions to facilitate deeper imaging
- Quadralign 4-axis auto-alignment optics provides both 2 horizontal axes (X & Y) and 2 angular axes (Olympus-exclusive) of automatic laser realignment for precise multicolor imaging and precise co-alignment with two-laser systems
- Prior z-deck linear-encoded motorized x-y stage with z-height adjustment
- Possibility for upgrade to carry out in vivo electrophysiology
Tandem Scanners
- Olympus SIM scanner for simultaneous imaging and photostimulation
- Olympus-patented SIM scanner provides additional galvanometer scanning unit for region-of-interest stimulation simultaneous to imaging for optogenetic stimulation, uncaging, etc.
- Wide choice of stimulation modes enable users to select point stimulation, rastered regions-of- interest, or Tornado (spiral) scans for high-efficiency stimulation
Light Sources
- Main scanner laser–Spectra Physics INSIGHT DS+ -OL pulsed IR LASER, tunable from 680 nm to 1300 nm, 120 fs pulse width at specimen plane
- Stimulation laser–Olympus-specific SPECTRA PHYSICS MAI TAI HP DEEP SEE-OL pulsed IR LASER, tunable from 690 nm to 1040 nm, 100 fs pulse width at specimen plane
- Olympus HGLGPLS widefield fluorescence illumination light source 130 W
Conventional Fluorescence Filters
- U-FBVW; blue-violet excitation, 400-440/455/460 LP
- U-FBNA; blue excitation, 470-495/505/510-550 BP
- U-FGW; green, 530-550/570/575 LP
Deep Focus Mode improves image brightness (23 slice MIPs)
Objectives
- XLPLN10XSVMP, Olympus ultra-long working distance 10X MPE-optimized multi-immersion objective 0.60NA, WD 8 mm, correct collar for refractive indices 1.33-1.52
- XLPLN25XWMP2, Olympus ultra 25x MPE water-immersion objective 1.05 NA, 2 mm WD
- 5x NA 0.1, WD 20 mm dry (MPLN5X-1-7) and 40x, NA 0.80, WD 3.30 mm water-immersion (LUMPLFLN40XW(F)) objectives on swing nosepiece
Optimized for best live-tissue and intravital multiphoton imaging. Widefield design maximizes fluorescence light capture. Correction collar provides correction for spherical aberration across a wide variety of tissue refractive indices 400nm-1600nm transmission coatings. Chromatically corrected for 700nm to 1300nm IR lasers.
Dichroic(s)
- AOBS serves as tunable dichroic for all lasers
Detectors
- Includes 2 channels of GaAsP PMTs for maximum sensitivity and 2 channels of multi-alkali PMTs for increased spectral range
- Transmitted light detector for Brightfield/DIC Image Acquisition
Olympus GaAsP detectors provide approximately twice the quantum efficiency of standard multi-alkali PMTs. All non-descanned detectors are positioned as close as possible to the specimen and signaling optics have been enlarged to facilitate maximum detection efficiency of scattered fluorescence.
Computer and Software
- HP Windows 7 64-bit Workstation and 30” Monitor, 2TB HD, 128 & 256 GB SSDs, 32GB DDR3 RAM
- FVMPE-RS system software including sequence manager, stage control, multipoint mapping, and data analysis
- Full-feature acquisition software provides complete control of system components including laser wavelength and intensity attenuation, laser coupling optics, scanners, & PMTs
- Stage Control Software provides XY motorized stage control, map image acquisition for easy target locating, tiling acquisition, and image stitching
- Multipoint Mapping Software provides high-speed multipoint stimulation, multipoint acquisition, and optogenetic mapping
- Sequence Manager allows programming of complex imaging tasks with microsecond precision within imaging tasks and millisecond precision between imaging tasks for up to two weeks
- Embedded 64-bit 3D rendering tool
- Olympus cellSens Dimension provided for post-acquisition data analysis
Tutorials

Environmental Scanning Electron Microscope, Beam: 30 keV (W filament), High resolution (3nm)/high vacuum mode, Variable pressure mode (10-3000 Pa) for non-conductive specimen, Detectors: 3 SE, 1 BSD, XRF Deben peltier cooling stage (-25° to +50°) for wet samples

You must be logged in to post a comment.